-
Borosilicate vs. Soda Lime Glass: A Detailed Comparison
Understanding the Contrast: Borosilicate and Soda Lime Glass
In materials science, borosilicate and soda lime glasses occupy unique positions, offering distinct properties that make them useful across various applications. These differences primarily stem from their contrasting chemical compositions – while borosilicate glass integrates boron trioxide into its molecular structure, soda lime glass does not include boron-based elements.
An In-depth Look at Soda Lime Glass
Soda lime glass, with silicon dioxide as its main ingredient, is the most prevalent type of glass available today. Valued for its affordability, chemical stability, and robust physical characteristics such as hardness and adaptability, a unique feature of soda lime glass is its ability to be recycled; it can be melted and reshaped multiple times without significantly losing quality.
The production process of soda lime glass involves various raw materials, including sodium carbonate, lime, dolomite, silicon dioxide, and aluminum oxide. These are combined and melted in a glass furnace that can reach temperatures up to 1675 °C. The selection of raw materials can also affect the final color of the glass, with components like iron oxide producing green and brown glassware.
Soda lime glass, with its property of increasing viscosity as temperature decreases, can be easily shaped into a variety of forms. Manufacturers commonly use it for container and flat glass, each with unique applications.
Delving into Borosilicate Glass
Unlike soda lime glass, Borosilicate glass combines silicon dioxide and boron trioxide as its main glass-forming components. It is primarily known for its low coefficient of thermal expansion, providing exceptional resistance to thermal shock. These attributes make borosilicate glass the material of choice for producing laboratory equipment, such as reagent bottles and heat-resistant bakeware.
Borosilicate glass production involves melting boric oxide, silica sand, soda ash, and alumina. There are several types of borosilicate glass, including non-alkaline-earth borosilicate glass, alkaline-earth-containing borosilicate glass, and high borate borosilicate glass, each with characteristics determined by the specific raw materials used.
Differentiating Between Soda Lime and Borosilicate Glass
Although soda lime and borosilicate glasses primarily consist of silicon dioxide, their chemical compositions and the raw materials used in their creation vary significantly. Soda lime glass does not include boron-based components, whereas borosilicate glass incorporates boron trioxide.
Soda lime glass, comprised of sodium carbonate, lime, dolomite, silicon dioxide, and aluminum oxide, exhibits lower thermal resistance than borosilicate glass. Borosilicate glass, created from boric oxide, silica sand, soda ash, and alumina, features a remarkably low coefficient of thermal expansion, providing it with significant thermal shock resistance.
These differences in thermal resistance often dictate the specific uses of each type of glass. The choice between soda lime glass and borosilicate glass hinges on the application’s specific requirements.
Examining the Degradation of Borosilicate and Soda Lime Glass in Water
Studies focusing on the chemical degradation of soda lime and borosilicate glass reveal that borosilicate glass demonstrates a notably higher resistance to disintegration. These findings hold in slightly acidic (pH 6) and more alkaline environments (pH 10). In tests, the degradation rate of soda lime glass proved to be ten times greater than that of borosilicate glass. The significant difference in degradation resistance begins to manifest at 134°C, the starting temperature in the experiment.
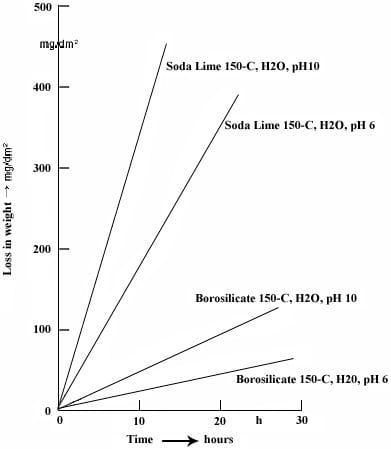
Translated from “VGB KRAFTWORKSTECHNIK”, Dr. A. Peters, Feb. 1979
Concluding Remarks: Borosilicate vs. Soda Lime Glass
A versatile material, glass is used extensively in domestic, laboratory, and industrial environments. The critical distinction between soda lime and borosilicate glass is their chemical structures. It lacks boron-based constituents, while borosilicate glass features boron trioxide as a significant component. This divergence in composition gives rise to their attributes, making each type of glass uniquely suited to different applications.