A recent article in Food Processing highlighted the importance of a master sanitation schedules. Essentially this schedule serves as a “back-up” to your daily maintenance procedures but involves the periodic cleaning and care for equipment and infrastructure inaccessible to daily cleaning activities.
The article covers two essential parts of the master sanitation schedule – the creation of the schedule and validating the procedures outlined in the schedule.
Schedule Creation
The starting point of creating the schedule, according to the article, is identifying where in your process you have contamination risk. Where are parts that could become a risk, either from poor design, material composition, process requirements, or other factors? Once identified, these non-compliance risks should be analyzed, and a set of cleaning requirements determined and added to the master sanitation schedule.
Schedule Validation
The practical application of the schedule requires validation. The article lays out a method that involves disassembly to reach the parts and visual inspection prior to taking samples both before and after the outlined cleaning. In this way, possible contaminants can be identified, and the cleaning procedure’s effectiveness and frequency are ensured.
Sight Glasses – A Window Into Your Master Sanitation Schedule
It stands to reason that the more parts that are a contamination risk and not readily accessible, the longer your master sanitation schedule, and the more production time lost to accessing and cleaning those parts. However, using accessible and hygienically designed equipment can reduce the number of items that could pose a contamination risk and reduce the time and effort needed to address them in the master sanitation schedule.
LJ Star has several products that fit those criteria.
Our sanitary sight glasses let you see more parts of your process, eliminating unknown corners that could become a concern while offering improved hygienic operation. Properly installed sanitary fittings make disassembly easier to perform and are less likely to add to contamination risks. In addition, LJ Star offers flow indicators, sight windows, bubble traps, and other sanitary products designed to meet various hygienic standards, along with the documentation and traceability to ensure they are, when properly maintained, the products that match your precise specifications.
The LJ Star Resource Center has many tools to help you and your company ensure sanitary standards.
Twinsburg, OH – February 22, 2021 – LJ Star offers a line of Anti-Galling Clamps that are specially designed to prevent the adhesion of materials due to friction and to keep processes running smoothly. The full line of Anti-Gall Clamps is produced to the highest hygienic engineering standards and are constantly being updated based on customer feedback and industry requirements.
“If galling occurs on a sanitary clamp, the nut and bolt fuse together so the operator can’t easily remove the clamp,” explained LJ Star National Sales Manager Jeremy Sheldon. “Quite often, the operator would have to cut off the clamp to remove it. This would
 Eliminate Clamp Galling
Eliminate Clamp Galling
Pharmaceutical applications require hygienic environments. Companies involved in pharmaceutical production must ensure tight controls on product purity through every stage of the development process, from manufacturing and quality control to packaging and shipping. Due to the tight restrictions that the FDA and other
 Eliminate threads and gasket compression problems that lead to contamination with this innovative new solution from LJ Star. Designed for hygienic/sterile applications in pharmaceutical, food/beverage, brewery and biotech industries, the new Clamp Type Sterile Visual Flow Indicator, or CT-SVFI, uses a patent-pending design to prevent the o-ring and gasket compression issues that lead to contamination associated with conventional screwed or threaded fittings.
Eliminate threads and gasket compression problems that lead to contamination with this innovative new solution from LJ Star. Designed for hygienic/sterile applications in pharmaceutical, food/beverage, brewery and biotech industries, the new Clamp Type Sterile Visual Flow Indicator, or CT-SVFI, uses a patent-pending design to prevent the o-ring and gasket compression issues that lead to contamination associated with conventional screwed or threaded fittings.
Keeping a Firm Grip on Sanitary Clamp Safety
For pharmaceutical and biotech companies, maintaining sterile process control is an ever-increasing challenge – one that can lead to cross-contamination, short term loss of product, long term drug shortages, lawsuits and even loss of life should sterile process be breached.
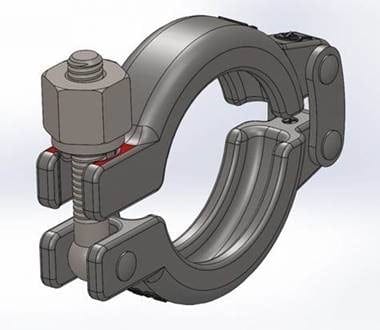 In our efforts to continually improve our products we are introducing a change to the Domed Hexagon Nut used to tighten our range of clamps. These nuts are used to replace the Wing Nut in certain applications. The existing Domed Hexagon Nut, we currently supply, will become obsolete to be replaced by the new Hexagon Flat Nut.
In our efforts to continually improve our products we are introducing a change to the Domed Hexagon Nut used to tighten our range of clamps. These nuts are used to replace the Wing Nut in certain applications. The existing Domed Hexagon Nut, we currently supply, will become obsolete to be replaced by the new Hexagon Flat Nut.
Gabe Montgomery, Engineering Manager, Tank Components Industries, an L.J. Star subsidiary located in Springfield, Missouri, is one of several L. J. Star engineers to serve on important ASME Bioprocessing Equipment (BPE) Committees. His work on the committee is part of the company’s contribution to advancing the technologies that shape our industry and to keep our customers informed on changes in the field. BPE held several meetings in San Diego, California in May 2018, and Gabe reports back on what he heard:
 For my pharmaceutical and biotech customers, sanitary clamps (also called hygienic clamps) keep systems clean while allowing quick disconnects. However, making reliable process system connections is anything but routine. If it isn’t done just right,
For my pharmaceutical and biotech customers, sanitary clamps (also called hygienic clamps) keep systems clean while allowing quick disconnects. However, making reliable process system connections is anything but routine. If it isn’t done just right,
Why Preventing Sanitary Clamp Galling Is Crucial to Product Purity
All makers of pharmaceuticals and biotechnology products have many concerns in common, but none is more critical than maintaining tight control over product purity during manufacturing and packaging. For example, if contamination were to compromise the integrity of a single batch of insulin, it could easily mean a loss of hundreds of thousands or millions of dollars in revenue to the manufacturer.
Recently, managers at a major U.S. pharmaceutical manufacturer were reminded of the importance of constant vigilance in their containment systems when they discovered leaks in sanitary clamp connections that forced them to scrap costly process media.
In a pharmaceutical or biotechnology setting, process equipment and piping are connected using a stainless steel sanitary clamp that forces together two ferrules, whose raised lips on the pipe ends facilitate a tight connection. Sanitary fittings are made specifically for pharmaceutical applications, where joint cleanliness and access are of paramount importance. Clamps allow quick disassembly for cleaning and sterilization. To prevent the formation of shelves or pockets that can harbor bacteria the ferrules, gasket and clamp must also provide a non-protruding, recess-less product contact surface.
Any plant operator can tell you that an innocent-looking set of pipes can conceal nasty secrets, some as petrifying as the monster hiding in the plumbing in a Harry Potter movie. A sight flow indicator is a good way to discover what’s lurking within.
These simple, low-cost devices provide a visual means of verifying liquid flow for direction and approximate rate, and also to observe the color and clarity of process fluids. The body of a sight flow indicator is equipped with one or more viewing windows, usually with gaskets, and a way to mount the indicator to the pipeline, such as a flanged, threaded, or sanitary clamp fitting. They are available to fit standard pipe sizes ranging from ¼-inch to 16 inches and carry ANSI pressure ratings.